Table of Contents
ToggleCHARGED PARTICLES IN MATTER
The presence of charged particles in matter is suggested by the phenomena of static electricity and electricity conduction through certain substances.
Therefore, Atoms can be divided further into sub-atomic particles.
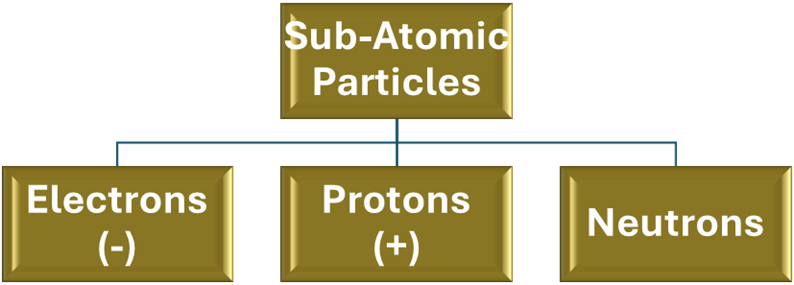
*SUB-ATOMIC PARTICLES
An atom is the smallest unit of an element that still retains its chemical properties. It consists of three fundamental sub-atomic particles:
Electrons (e−): These are the negatively charged particles having very little mass. They orbit the nucleus.
Protons (p+): These are the positively charged particles. They are located in the nucleus and have a mass of about one atomic mass unit.
Neutrons (n): These are the particles that are neutral (have no charge). They are also found in the nucleus and have a mass roughly equivalent to that of a proton.
EARLY MODELS OF ATOM
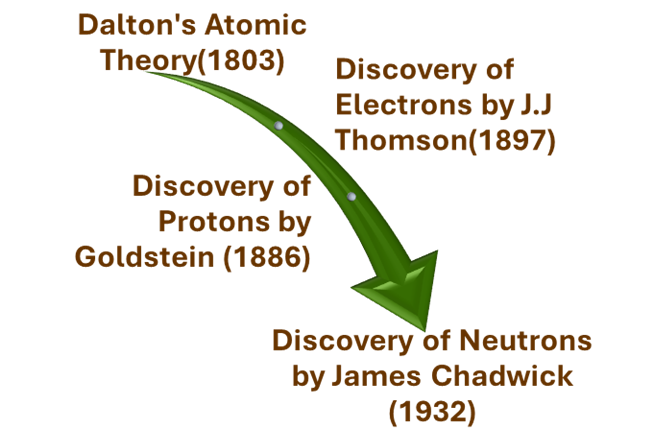
*DALTON’S ATOMIC THEORY
Dalton’s theory states that matter is made up of small indestructible atoms that combine in set ratios and rearrange in reactions without being created or destroyed.
This theory suggested that Atom is indivisible – which could not be broken down into smaller particles.
# But the discovery of Sub-Atomic Particles inside the atom disproved this principle of Dalton’s atomic theory.
*DISCOVERY OF ELECTRONS (1897)
-Given by: J.J. Thomson in 1897.
-J.J. Thomson discovered the electron in 1897 through the cathode ray experiment, proving atoms are not indivisible.
-Experiment Setup
- A glass tube with very low-pressure gas.
- High voltage applied between cathode (–) and anode (+).
- Observed rays coming from cathode → called cathode rays.
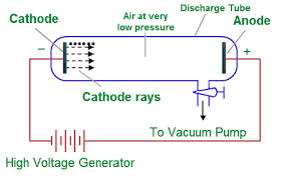
-Key Observations
- Rays travel in straight lines.
- Deflected by electric and magnetic fields → showed they are negatively charged particles.
- Could move a light paddle wheel → proved they have mass.
-Conclusion
- Cathode rays are made of tiny negatively charged particles → Electrons.
- First subatomic particle discovered.
-Thomson’s Contribution
- Proposed that atoms are divisible.
- Gave the Plum Pudding Model: atom is like a sphere of positive charge with electrons embedded (like seeds in watermelon).
-Characteristics of an Electron
- Mass of an Electron = 9.109 × 10⁻³¹ kg
- Charge of an Electron = 1.602 x 10-19 C
- Relative Charge on Electron = -1
*DISCOVERY OF PROTON
-Given by: E. Goldstein in 1886
–E. Goldstein discovered protons in 1886 through canal rays, identifying them as positively charged particles inside atoms.
-Experiment Setup
- When high voltage was applied to gases at low pressure in a discharge tube, besides cathode rays, new rays were observed moving in the opposite direction (from anode to cathode).
- These rays were called canal rays or anode rays.
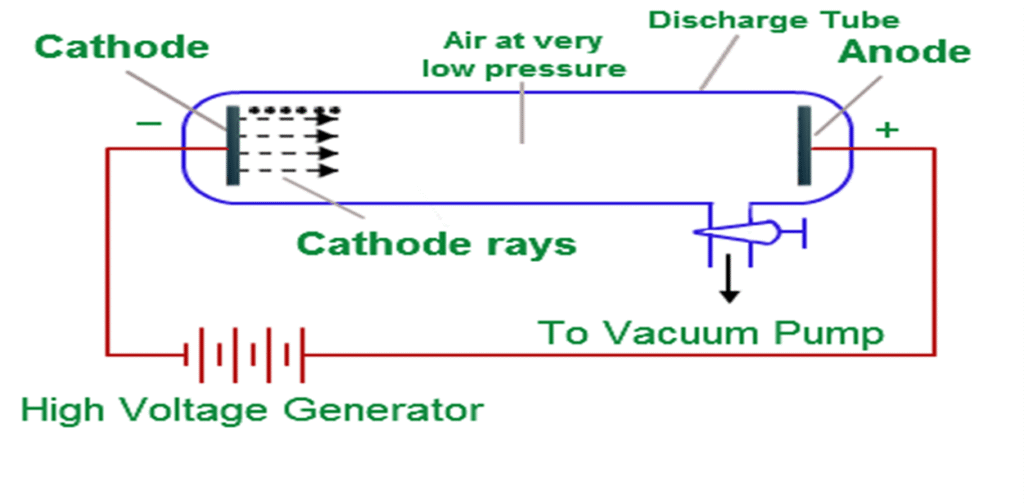
-Key Observations
- Canal rays travel in straight lines.
- They are deflected by electric and magnetic fields, but in the opposite direction to cathode rays → showing they are positively charged.
- The mass of these particles depended on the gas used, but the lightest and fundamental positive particle was identified in hydrogen.
-Conclusion
- The positively charged particle of hydrogen was named Proton.
- Mass of proton ≈ 1836 times that of an electron.
-Importance
- Discovery of proton showed that atoms contain positive charge too.
- Helped in developing the structure of atom further.
-characteristics of proton
- Mass of proton = 1.6726 × 10⁻²⁷ kg
- Charge of proton = 1.602 x 10⁻¹⁹ coulombs (c)
- Relative charge on proton = +1
*DISCOVERY OF NEUTRON
– Given by: James Chadwick
-In 1932, James Chadwick discovered the neutron, a neutral particle in the nucleus with mass equal to a proton.
-Experiment
- Chadwick bombarded beryllium with alpha particles.
- This released neutral radiation, which could knock out protons from substances like paraffin wax.
Key Observations
- The new radiation had no charge (not deflected by electric or magnetic fields).
- It had a mass nearly equal to that of a proton.
-Conclusion
- The neutral particle inside atoms was discovered and named Neutron.
- Neutrons are present in the nucleus along with protons.
-Importance
- Explained the remaining mass of the atom.
- Discovery of neutrons gave a complete picture of the atom’s nucleus.
-Characteristics of Neutron
- Mass of neutron = 1.675 × 10⁻²⁷ kg
- Charge of Neutron = A neutron has no electric charge, it is an electrically neutral.
ATOMIC MODEL
There are three Atomic Models on Arrangement of Sub-Atomic Particles.

1) Thomson’s Model of the Atom (1898)
-After discovering the electron, J.J. Thomson proposed a model to explain the structure of the atom.
-Thomson imagined the atom as a sphere of positive charge with electrons studded inside it, called the Plum Pudding Model.
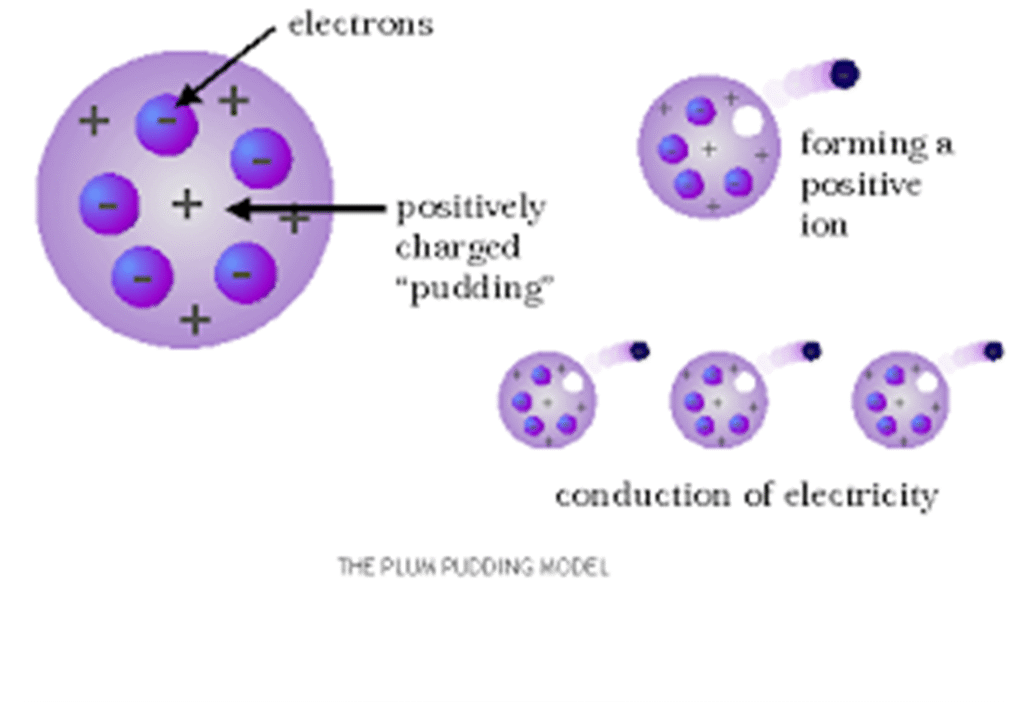
-Main Features
- Spherical Atom – Atom is a positively charged sphere.
- Embedded Electrons – Negatively charged electrons are embedded in it, like “seeds in a watermelon” or “plums in a pudding.”
- Neutral Atom – Positive charge of the sphere balances the negative charge of electrons, so the atom is electrically neutral.
-Limitations
- Could not explain the results of Rutherford’s alpha particle scattering experiment.
- Failed to explain the exact arrangement of electrons.
-Importance
- First scientific attempt to explain atomic structure.
- Introduced the idea that atoms are divisible and contain subatomic particles.
2) Rutherford’s Model (Nuclear Model)
-Rutherford performed the Gold Foil Experiment (α-particle scattering experiment) with Geiger and Marsden.
-Aim: To test Thomson’s Plum Pudding Model.
-Experiment Setup
- A thin gold foil (few atoms thick).
- Fast-moving alpha particles (positively charged) bombarded on it.
- A fluorescent screen around to detect scattering.
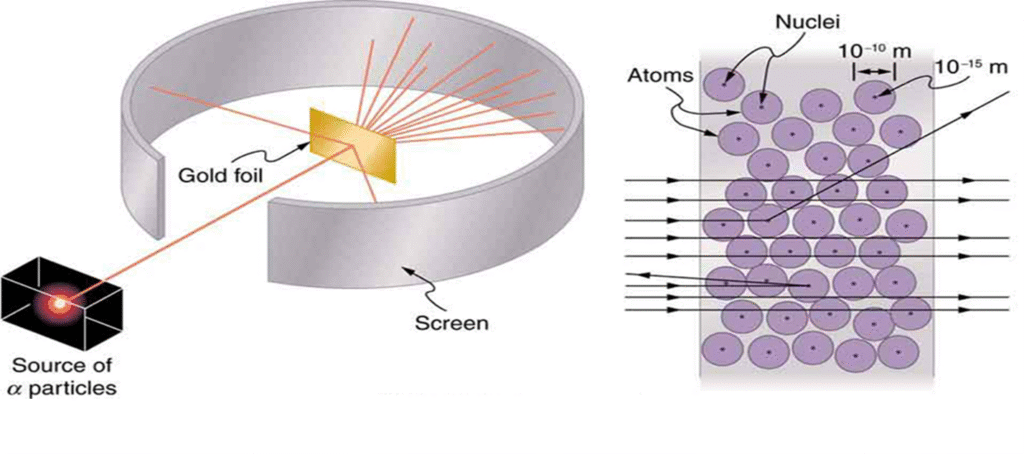
-Observations
- Most α-particles passed through → atom has large empty space.
- Some deflected at small angles → positive charge is concentrated.
- Very few (1 in 8000) bounced back → there is a tiny, dense, positive nucleus.
-Main Features of Rutherford’s Model
- Atom has a small, dense, positively charged nucleus at the center.
- Almost all mass of the atom is in the nucleus.
- Electrons revolve around the nucleus in circular orbits (like planets around the sun).
- Most of the atom is empty space.
-Limitations
- Could not explain the stability of atom (according to physics, revolving electrons should lose energy and fall into nucleus).
- Could not explain atomic spectra.
-Importance
- First to show existence of nucleus.
- Paved the way for Bohr’s Model.
3) Bohr’s Model
-Niels Bohr improved Rutherford’s model.
-Solved the stability problem and explained hydrogen spectrum.
-Postulates of Bohr’s Model
- Electrons revolve in fixed paths
- Electrons move in definite circular orbits called shells/energy levels around the nucleus.
- These orbits have fixed energy.
- Energy levels are quantized
- Only certain orbits (K, L, M, N …) are allowed.
- Each level is represented by a quantum number (n = 1, 2, 3 …).
- No energy loss in orbit
- While revolving in a fixed orbit, electrons do not radiate energy, so the atom is stable.
- Energy absorption or emission
- Electrons can jump from one orbit to another:
- Jump to higher orbit → absorbs energy.
- Jump to lower orbit → emits energy (in form of light of definite wavelength).
- Electrons can jump from one orbit to another:
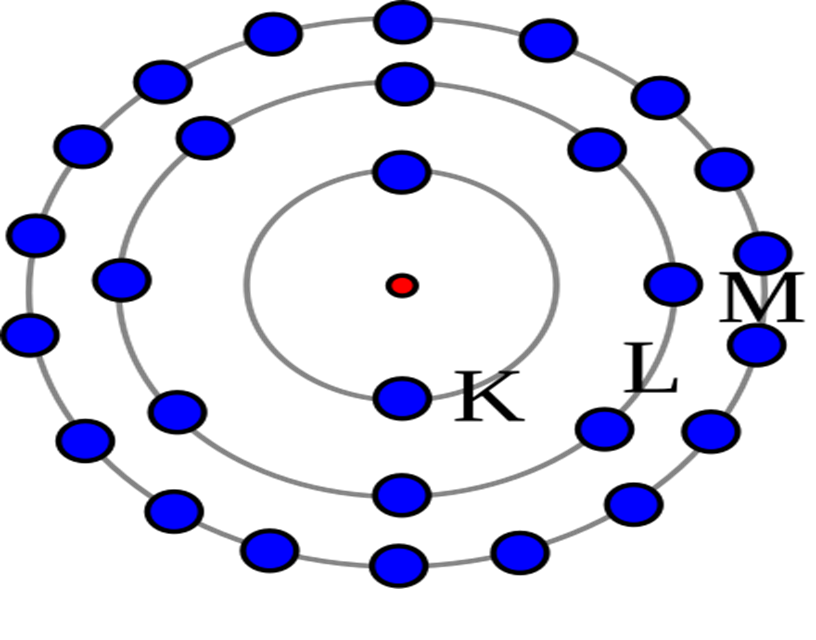
-Achievements
- Explained the hydrogen line spectrum successfully.
- Solved Rutherford’s problem of atomic collapse.
–Limitations
- Couldn’t explain spectra of multi-electron atoms.
- Couldn’t explain fine details like splitting of lines (Zeeman effect, Stark effect).
- Quantum mechanics later replaced it.
Atomic Number and Mass Number
An atom is made up of subatomic particles: protons, neutrons, and electrons. The atomic number and mass number are two important terms used to describe the composition of an atom.
*Atomic Number (Z)
The atomic number, symbolized by Z, is the number of protons in the nucleus of an atom.
- Each element has a unique atomic number, which acts like its identity card. For example, every atom of carbon has exactly 6 protons.
- In a neutral atom, the number of protons is equal to the number of electrons. Therefore, the atomic number also tells you the number of electrons.
- Atomic number decides the place of an element in the periodic table.
Formula:
Atomic Number(Z)=Number of Protons
For a neutral atom:
Number of Electrons=Number of Protons
*Mass Number (A)
The mass number, symbolized by A, is the total number of protons and neutrons in the nucleus of an atom. Since the mass of electrons is negligible, the mass number essentially represents the total mass of the atom’s nucleus.
- Protons and neutrons are collectively known as nucleons.
- The mass number is always a whole number.
- Mass number helps to differentiate isotopes of the same element.
Formula:
Mass Number(A)=Number of Protons + Number of Neutrons
You can rearrange this formula to find the number of neutrons:
Number of Neutrons = Mass Number(A) – Atomic Number(Z)
*Isotope Notation
Elements are often represented with their atomic and mass numbers. The notation is as follows:
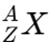
Where:
- A is the Mass Number (superscript)
- Z is the Atomic Number (subscript)
- X is the chemical symbol of the element
Example: Carbon (C) A common isotope of carbon has an atomic number of 6 and a mass number of 12. This can be written as:
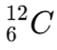
- Atomic Number (Z = 6): This means carbon has 6 protons.
- Mass Number (A = 12): This means it has a total of 12 protons and neutrons.
- Number of Neutrons: 12−6=6
Electron Distribution in Orbits
After the discovery of protons, electrons, and neutrons, the next task was to understand how electrons are arranged within an atom.
-Niels Bohr and Bury developed laws for the distribution of electrons in different shells (orbits/energy levels) surrounding the nucleus.
*Rules for Electron Distribution
1. Naming of Shells
- Shells are denoted by letters:
- K, L, M, N … (starting from nucleus).
- Represented by quantum numbers (n):
- K = 1, L = 2, M = 3, N = 4 …
2. Maximum Number of Electrons in a Shell
- Formula: 2n²
- K shell (n=1) → 2 × 1² = 2 electrons
- L shell (n=2) → 2 × 2² = 8 electrons
- M shell (n=3) → 2 × 3² = 18 electrons
- N shell (n=4) → 2 × 4² = 32 electrons
3. Octet Rule (Stability Rule)
- The outermost shell of an atom can hold a maximum of 8 electrons.
- Atoms try to achieve 8 electrons in their outermost shell to become stable (like noble gases).
4. Filling of Electrons in Successive Shells
- Electrons fill shells in order of increasing energy:
- First K, then L, then M, then N …
- The outermost shell cannot have more than 8 electrons, and the next inner shell cannot have more than 18 electrons.
*Importance of Electron Distribution
- Determines chemical properties of an element.
- Decides valency (combining capacity).
- Explains reactivity of metals and non-metals.
- Helps in understanding the periodic table arrangement.
VALENCY
Valency is the combining capacity of an atom. It is the number of electrons an atom must gain, lose, or share to achieve a stable electronic configuration, typically having a full outermost shell (an octet of 8 electrons, or a duplet of 2 for elements like Helium). This stable state resembles the electronic configuration of a noble gas.
- Atoms react to become stable. They achieve this stability by forming bonds with other atoms.
- Valency is a whole number and has no positive or negative sign.
-Atoms are stable when they have 8 electrons in their outermost shell (Octet Rule).
-To become stable, atoms:
- Lose electrons (if 1, 2, or 3 in outer shell → metals).
- Gain electrons (if 5, 6, or 7 in outer shell → non-metals).
- Share electrons (as in covalent compounds like H₂, O₂).
*How to Determine Valency
The valency of an element is determined by the number of electrons in its outermost shell, known as valence electrons.
- For elements with 1, 2, 3, or 4 valence electrons: The valency is simply equal to the number of valence electrons.
- Example: Sodium (Na)
- Electronic configuration: 2, 8, 1
- It has 1 valence electron. It can easily lose this one electron to become stable.
- Valency = 1
- Example: Sodium (Na)
- For elements with 5, 6, or 7 valence electrons: The valency is calculated by subtracting the number of valence electrons from 8.
- Example: Oxygen (O)
- Electronic configuration: 2, 6
- It needs 2 more electrons to complete its octet (8 – 6 = 2).
- Valency = 2
- Example: Oxygen (O)
*Types of Valency
Based on how atoms achieve stability, we can distinguish between two types of valency:
- Electro valency: This refers to the number of electrons lost or gained by an atom to form an ionic bond. It results in the formation of charged particles called ions.
- Covalency: This refers to the number of electrons shared by an atom to form a covalent bond.
*Noble Gases: Zero Valency
Noble gases (Group 18 elements) like Helium, Neon, and Argon have a completely filled outermost shell (a stable octet or duplet). Because they are already stable, they do not need to gain, lose, or share electrons.
#Valency of noble gases is zero. They are generally unreactive.
*Importance of Valency
- Explains how elements combine to form compounds.
- Helps in writing chemical formulas.
- Example: Na (1) + Cl (1) → NaCl
- Mg (2) + Cl (1) → MgCl₂
ISOTOPES AND ISOBARS
*Isotopes
-Definition
- Isotopes are atoms of the same element having the same atomic number (Z) but different mass numbers (A).
- Difference arises due to different number of neutrons.
– Characteristics
- Same chemical properties (since same number of electrons/protons).
- Different physical properties (due to different mass).
– Example: Chlorine has two isotopes, Chlorine-35 and Chlorine-37.
- Chlorine-35: 17 protons, 18 neutrons.
- Chlorine-37: 17 protons, 20 neutrons.
– Uses of Isotopes
- U-235: nuclear fuel.
- C-14: radiocarbon dating.
- I-131: treatment of thyroid disease.
*Isobars
– Definition
- Isobars are atoms of different elements having the same mass number (A) but different atomic numbers (Z).
- Difference arises due to different number of protons and neutrons, but sum remains same.
– Characteristics
- Different chemical properties (since different atomic numbers).
- May have similar physical mass-related properties.



Best notes